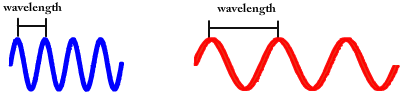
Visible light comes in many different colors. What makes each color unique is its wavelength. The word wavelength means the distance between wave crests.

Notice that blue light has a much shorter wavelength (the crests are closer together) than red light. This is how your eyes can tell them apart. There are special cells inside your eye that only respond to specific colored wavelengths.
If you drag the handle on the wave below you can stretch the wave longer or squish the wave shorter. Notice as you do so that the color of the waves change.
Which color of light has the longest wavelength?
Which color of light has the shortest wavelength?
Which color of light has a longer wavelength: green or yellow?
Which color of light has a shorter wavelength: blue or orange?
If lots of different colors hit your eye at the same time your brain interprets it as "white" light. This is like the light coming from the sun or the light from a light bulb. Even though you might associate the color white as having "no color" at all. White light actually has ALL the colors inside of it. You can split the colors apart by shining them through a device called a prism
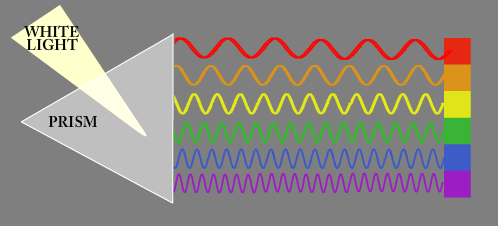
A prism separates light based on wavelength. Notice that all the long waves (red) end up on one side, and all the short waves (violet) end up on the other side. This is why a rainbow always appears in the same color order!
When an atom gets hot enough it will start to glow. But it does NOT emit the entire rainbow. It only emits certain wavelengths. Notice in the picture below that hydrogen only emits a small portion of the total spectrum.
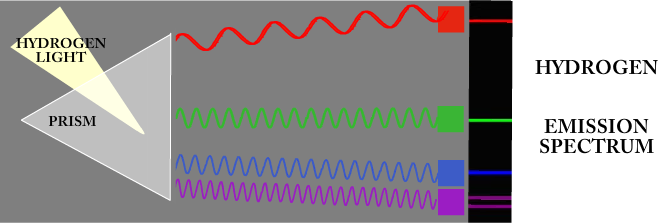
When you pass light from hydrogen into a prism you get five very distinct color bands. This is like a color fingerprint. It allows you to identify hydrogen because no other atom emits exactly the same color combination.
In order to analyze the colors an object is emitting you need to use a device called a spectrometer. Let's look at this word in more detail.
The word spectro is the same as spectrum. It means a range of colors.
The word meter means to measure.
So literally, a spectrometer is a device that measures colors.
The ions in the Flame Test Lab were easy to distinguish because their colors were so different. But what if there are two ions that both emit the color red? How could you tell them apart? If you only could use your eyes, it would be very difficult. But with a spectrometer it is possible. Every shade of every color has a unique wavelength. Even though two shades of red may look identical to the human eye, a spectrometer can tell them apart.
A spectrometer uses a prism (like in the picture above). Each color that leaves the prism is deflected by a different angle. Red is deflected the most and violet is deflected the least. The detector can measure the angle of deflection and calculate the exact wavelengths. The result is a sequence of colored stripes that is unique for every atom. Each stripe on the emission spectrum corresponds to a very specific wavelength.
Imagine that light from two distant stars is coming to the Earth. Scientists want to know what the stars are made of. They are too far away to send astronauts or probes to. What can they do? They can analyze the light. Look at the pictures below.
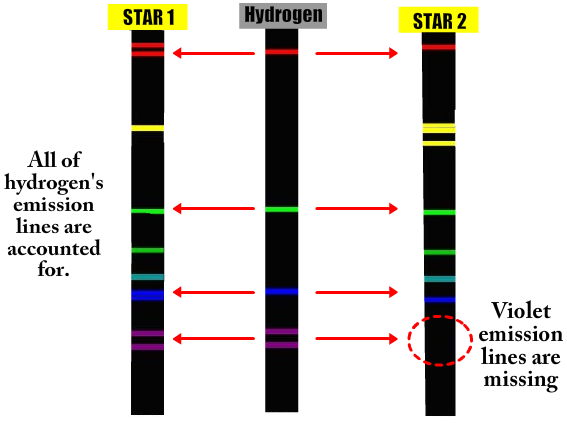
Notice that Star 1 is emitting ALL of hydrogen's spectrum lines. This means that Star A must contain hydrogen. But what are all the extra lines? A star is not just made of one element. In addition to Hydrogen, there are other atoms in the star that are emitting light. By comparing the extra lines with other known emission spectrum you could identify what the other element is.
At first glance Star 2 looks like a good match to hydrogen as well. But once we get to the bottom there is a problem. The violet lines are completely missing. This means that Star 2 must NOT contain hydrogen.
Let's try applying what you just learned. The diagram shown below has seven large stars that have been numbered. The red circle represents what you are currently viewing through your telescope.


| STEP 1: | Drag the red circle to the star you wish to analyze. |
| STEP 2: | The light from your star passes through the telescope into a spectrometer that shows you an emission spectrum. |
| STEP 3: | Compare the emission spectrum with the known elements to determine what the star is made out of. (To make this easier, the named elements can be dragged and lined up with the star's emission spectrum.) |
EMISSION SPECTRUM
DRAG ME NEXT TO EMISSION SPECTRUM TO COMPARE SPECTRAL LINES


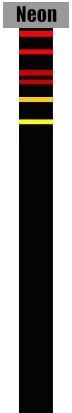


Identify the elements found in each star. It is possible to have more than one element in the same star.
| Helium | Hydrogen | Mercury | Neon | Sodium | |||
| Star 1 | |||||||
| Star 2 | |||||||
| Star 3 | |||||||
| Star 4 | |||||||
| Star 5 | |||||||
| Star 6 | |||||||
| Star 7 |